
HIV & AIDS Drug Discovery
Trajectory of Clinical Studies from Bench to Bedside
Public and private sector organizations support research that advances our understanding of HIV and how it causes disease, thereby unlocking new targets for drug development.
Promising medicines are then tested in human clinical trials to determine whether they are safe and effective.
This process usually takes several years to complete before a new therapy is available to the public.

HIV research is focused on finding new and more effective therapies, drug classes, and antiretroviral drug combinations that can extend and improve the quality of life for people living with HIV/AIDS.
What is Antiretroviral Therapy (ART)?
Treatment with HIV medicines is called ART.
People on ART take a combination of HIV medicines (called an HIV regimen) every day.
A person's initial HIV regimen generally includes three HIV medicines from at least two different drug classes.
ART can’t cure HIV, but HIV medicines help people with HIV live longer healthier lives.
HIV medicines also reduce the risk of HIV transmission.

Despite the availability of 31 approved antiretroviral drugs, there is continued interest in developing new agents for the treatment of HIV/AIDS for the following reasons:
1:An effective vaccine or comparable preventative is unlikely to become available for years, and the epidemic will therefore be sustained in endemic areas and increase in new regions where the virus is introduced.
2:There will be a need for better tolerated, more convenient and less expensive treatments.
3:Increasing resistance to existing drugs will prompt the search for agents in classes that do not share cross-resistance, and for new classes of drugs that would not be affected by such resistance.
As shown in the figure below:
a. The life cycle of HIV-1, showing the step at which the drug enfuvirtide acts, and also the steps targeted by other approved drugs (brown) and those in development (blue).
b. Simplified schematic of the mechanism by which enfuvirtide inhibits viral entry into host cells.
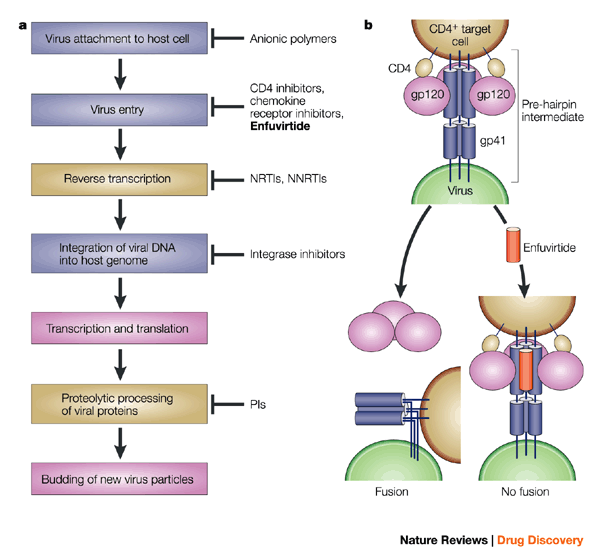
-
The envelope glycoprotein of HIV-1 consists of two non-covalently associated subunits, gp120 and gp41.
-
After attachment of HIV-1 to its target cells carrying the CD4 receptor, gp120 interacts with the CD4 receptor, which initiates a series of conformational changes in gp41 and gp120 that lead to the insertion of a region of gp41 into the membrane of the host cell, and the formation of a 'pre-hairpin intermediate' (top).
-
Further changes in the conformation of gp41 bring the viral and cellular membranes into close enough proximity for membrane fusion (bottom left).
-
Enfuvirtide binds to a region of gp41 that mediates this conformational change from pre-hairpin intermediate to the fusion-active structure, thereby preventing fusion and viral entry (bottom right). NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.